Ocean Acidification
Introduction
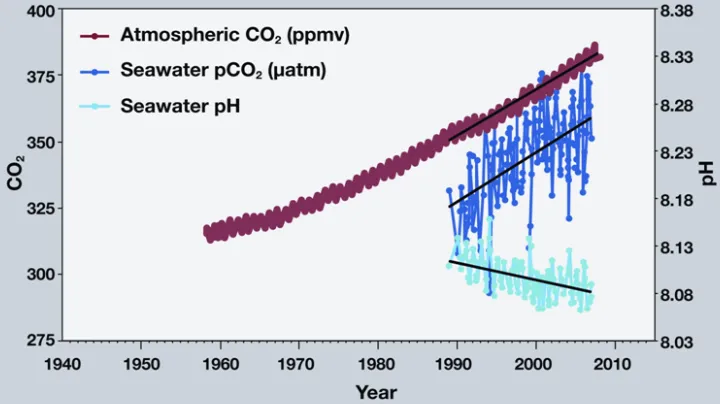
Ocean acidification is sometimes called “climate change’s equally evil twin,” and for good reason: it's a significant and harmful consequence of excess carbon dioxide in the atmosphere that we don't see or feel because its effects are happening underwater. At least one-quarter of the carbon dioxide (CO2) released by burning coal, oil and gas doesn't stay in the air, but instead dissolves into the ocean. Since the beginning of the industrial era, the ocean has absorbed some 525 billion tons of CO2 from the atmosphere, presently around 22 million tons per day.
At first, scientists thought that this might be a good thing because it leaves less carbon dioxide in the air to warm the planet. But in the past decade, they’ve realized that this slowed warming has come at the cost of changing the ocean’s chemistry. When carbon dioxide dissolves in seawater, the water becomes more acidic and the ocean’s pH (a measure of how acidic or basic the ocean is) drops. Even though the ocean is immense, enough carbon dioxide can have a major impact. In the past 200 years alone, ocean water has become 30 percent more acidic—faster than any known change in ocean chemistry in the last 50 million years.
Scientists formerly didn’t worry about this process because they always assumed that rivers carried enough dissolved chemicals from rocks to the ocean to keep the ocean’s pH stable. (Scientists call this stabilizing effect “buffering.”) But so much carbon dioxide is dissolving into the ocean so quickly that this natural buffering hasn’t been able to keep up, resulting in relatively rapidly dropping pH in surface waters. As those surface layers gradually mix into deep water, the entire ocean is affected.
Such a relatively quick change in ocean chemistry doesn’t give marine life, which evolved over millions of years in an ocean with a generally stable pH, much time to adapt. In fact, the shells of some animals are already dissolving in the more acidic seawater, and that’s just one way that acidification may affect ocean life. Overall, it's expected to have dramatic and mostly negative impacts on ocean ecosystems—although some species (especially those that live in estuaries) are finding ways to adapt to the changing conditions.
However, while the chemistry is predictable, the details of the biological impacts are not. Although scientists have been tracking ocean pH for more than 30 years, biological studies really only started in 2003, when the rapid shift caught their attention and the term "ocean acidification" was first coined. What we do know is that things are going to look different, and we can't predict in any detail how they will look. Some organisms will survive or even thrive under the more acidic conditions while others will struggle to adapt, and may even go extinct. Beyond lost biodiversity, acidification will affect fisheries and aquaculture, threatening food security for millions of people, as well as tourism and other sea-related economies.
Acidification Chemistry
At its core, the issue of ocean acidification is simple chemistry. There are two important things to remember about what happens when carbon dioxide dissolves in seawater. First, the pH of seawater water gets lower as it becomes more acidic. Second, this process binds up carbonate ions and makes them less abundant—ions that corals, oysters, mussels, and many other shelled organisms need to build shells and skeletons.
A More Acidic Ocean
Carbon dioxide is naturally in the air: plants need it to grow, and animals exhale it when they breathe. But, thanks to people burning fuels, there is now more carbon dioxide in the atmosphere than anytime in the past 15 million years. Most of this CO2 collects in the atmosphere and, because it absorbs heat from the sun, creates a blanket around the planet, warming its temperature. But some 30 percent of this CO2 dissolves into seawater, where it doesn't remain as floating CO2 molecules. A series of chemical changes break down the CO2 molecules and recombine them with others.
When water (H2O) and CO2 mix, they combine to form carbonic acid (H2CO3). Carbonic acid is weak compared to some of the well-known acids that break down solids, such as hydrochloric acid (the main ingredient in gastric acid, which digests food in your stomach) and sulfuric acid (the main ingredient in car batteries, which can burn your skin with just a drop). The weaker carbonic acid may not act as quickly, but it works the same way as all acids: it releases hydrogen ions (H+), which bond with other molecules in the area.
Seawater that has more hydrogen ions is more acidic by definition, and it also has a lower pH. In fact, the definitions of acidification terms—acidity, H+, pH —are interlinked: acidity describes how many H+ ions are in a solution; an acid is a substance that releases H+ ions; and pH is the scale used to measure the concentration of H+ ions.
The lower the pH, the more acidic the solution. The pH scale goes from extremely basic at 14 (lye has a pH of 13) to extremely acidic at 1 (lemon juice has a pH of 2), with a pH of 7 being neutral (neither acidic or basic). The ocean itself is not actually acidic in the sense of having a pH less than 7, and it won’t become acidic even with all the CO2 that is dissolving into the ocean. But the changes in the direction of increasing acidity are still dramatic.
So far, ocean pH has dropped from 8.2 to 8.1 since the industrial revolution, and is expected by fall another 0.3 to 0.4 pH units by the end of the century. A drop in pH of 0.1 might not seem like a lot, but the pH scale, like the Richter scale for measuring earthquakes, is logarithmic. For example, pH 4 is ten times more acidic than pH 5 and 100 times (10 times 10) more acidic than pH 6. If we continue to add carbon dioxide at current rates, seawater pH may drop another 120 percent by the end of this century, to 7.8 or 7.7, creating an ocean more acidic than any seen for the past 20 million years or more.
Why Acidity Matters
Many chemical reactions, including those that are essential for life, are sensitive to small changes in pH. In humans, for example, normal blood pH ranges between 7.35 and 7.45. A drop in blood pH of 0.2-0.3 can cause seizures, comas, and even death. Similarly, a small change in the pH of seawater can have harmful effects on marine life, impacting chemical communication, reproduction, and growth.
The building of skeletons in marine creatures is particularly sensitive to acidity. One of the molecules that hydrogen ions bond with is carbonate (CO3-2), a key component of calcium carbonate (CaCO3) shells. To make calcium carbonate, shell-building marine animals such as corals and oysters combine a calcium ion (Ca+2) with carbonate (CO3-2) from surrounding seawater, releasing carbon dioxide and water in the process.
Like calcium ions, hydrogen ions tend to bond with carbonate—but they have a greater attraction to carbonate than calcium. When a hydrogen bonds with carbonate, a bicarbonate ion (HCO3-) is formed. Shell-building organisms can't extract the carbonate ion they need from bicarbonate, preventing them from using that carbonate to grow new shell. In this way, the hydrogen essentially binds up the carbonate ions, making it harder for shelled animals to build their homes. Even if animals are able to build skeletons in more acidic water, they may have to spend more energy to do so, taking away resources from other activities like reproduction. If there are too many hydrogen ions around and not enough molecules for them to bond with, they can even begin breaking existing calcium carbonate molecules apart—dissolving shells that already exist.
This is just one process that extra hydrogen ions—caused by dissolving carbon dioxide—may interfere with in the ocean. Organisms in the water, thus, have to learn to survive as the water around them has an increasing concentration of carbonate-hogging hydrogen ions.
Impacts on Ocean Life
The pH of the ocean fluctuates within limits as a result of natural processes, and ocean organisms are well-adapted to survive the changes that they normally experience. Some marine species may be able to adapt to more extreme changes—but many will suffer, and there will likely be extinctions. We can't know this for sure, but during the last great acidification event 55 million years ago, there were mass extinctions in some species including deep sea invertebrates. A more acidic ocean won’t destroy all marine life in the sea, but the rise in seawater acidity of 30 percent that we have already seen is already affecting some ocean organisms.
Coral Reefs
Reef-building corals craft their own homes from calcium carbonate, forming complex reefs that house the coral animals themselves and provide habitat for many other organisms. Acidification may limit coral growth by corroding pre-existing coral skeletons while simultaneously slowing the growth of new ones, and the weaker reefs that result will be more vulnerable to erosion. This erosion will come not only from storm waves, but also from animals that drill into or eat coral. A recent study predicts that by roughly 2080 ocean conditions will be so acidic that even otherwise healthy coral reefs will be eroding more quickly than they can rebuild.
Acidification may also impact corals before they even begin constructing their homes. The eggs and larvae of only a few coral species have been studied, and more acidic water didn’t hurt their development while they were still in the plankton. However, larvae in acidic water had more trouble finding a good place to settle, preventing them from reaching adulthood.
How much trouble corals run into will vary by species. Some types of coral can use bicarbonate instead of carbonate ions to build their skeletons, which gives them more options in an acidifying ocean. Some can survive without a skeleton and return to normal skeleton-building activities once the water returns to a more comfortable pH. Others can handle a wider pH range.
Nonetheless, in the next century we will see the common types of coral found in reefs shifting—though we can't be entirely certain what that change will look like. On reefs in Papua New Guinea that are affected by natural carbon dioxide seeps, big boulder colonies have taken over and the delicately branching forms have disappeared, probably because their thin branches are more susceptible to dissolving. This change is also likely to affect the many thousands of organisms that live among the coral, including those that people fish and eat, in unpredictable ways. In addition, acidification gets piled on top of all the other stresses that reefs have been suffering from, such as warming water (which causes another threat to reefs known as coral bleaching), pollution, and overfishing.
Oysters, Mussels, Urchins and Starfish
Generally, shelled animals—including mussels, clams, urchins and starfish—are going to have trouble building their shells in more acidic water, just like the corals. Mussels and oysters are expected to grow less shell by 25 percent and 10 percent respectively by the end of the century. Urchins and starfish aren’t as well studied, but they build their shell-like parts from high-magnesium calcite, a type of calcium carbonate that dissolves even more quickly than the aragonite form of calcium carbonate that corals use. This means a weaker shell for these organisms, increasing the chance of being crushed or eaten.
Some of the major impacts on these organisms go beyond adult shell-building, however. Mussels’ byssal threads, with which they famously cling to rocks in the pounding surf, can’t hold on as well in acidic water. Meanwhile, oyster larvae fail to even begin growing their shells. In their first 48 hours of life, oyster larvae undergo a massive growth spurt, building their shells quickly so they can start feeding. But the more acidic seawater eats away at their shells before they can form; this has already caused massive oyster die-offs in the U.S. Pacific Northwest.
This massive failure isn’t universal, however: studies have found that crustaceans (such as lobsters, crabs, and shrimp) grow even stronger shells under higher acidity. This may be because their shells are constructed differently. Additionally, some species may have already adapted to higher acidity or have the ability to do so, such as purple sea urchins. (Although a new study found that larval urchins have trouble digesting their food under raised acidity.)
Of course, the loss of these organisms would have much larger effects in the food chain, as they are food and habitat for many other animals.
Zooplankton
There are two major types of zooplankton (tiny drifting animals) that build shells made of calcium carbonate: foraminifera and pteropods. They may be small, but they are big players in the food webs of the ocean, as almost all larger life eats zooplankton or other animals that eat zooplankton. They are also critical to the carbon cycle—how carbon (as carbon dioxide and calcium carbonate) moves between air, land and sea. Oceans contain the greatest amount of actively cycled carbon in the world and are also very important in storing carbon. When shelled zooplankton (as well as shelled phytoplankton) die and sink to the seafloor, they carry their calcium carbonate shells with them, which are deposited as rock or sediment and stored for the foreseeable future. This is an important way that carbon dioxide is removed from the atmosphere, slowing the rise in temperature caused by the greenhouse effect.
These tiny organisms reproduce so quickly that they may be able to adapt to acidity better than large, slow-reproducing animals. However, experiments in the lab and at carbon dioxide seeps (where pH is naturally low) have found that foraminifera do not handle higher acidity very well, as their shells dissolve rapidly. One study even predicts that foraminifera from tropical areas will be extinct by the end of the century.
The shells of pteropods are already dissolving in the Southern Ocean, where more acidic water from the deep sea rises to the surface, hastening the effects of acidification caused by human-derived carbon dioxide. Like corals, these sea snails are particularly susceptible because their shells are made of aragonite, a delicate form of calcium carbonate that is 50 percent more soluble in seawater.
One big unknown is whether acidification will affect jellyfish populations. In this case, the fear is that they will survive unharmed. Jellyfish compete with fish and other predators for food—mainly smaller zooplankton—and they also eat young fish themselves. If jellyfish thrive under warm and more acidic conditions while most other organisms suffer, it’s possible that jellies will dominate some ecosystems (a problem already seen in parts of the ocean).
Plants and Algae
Plants and many algae may thrive under acidic conditions. These organisms make their energy from combining sunlight and carbon dioxide—so more carbon dioxide in the water doesn't hurt them, but helps.
Seagrasses form shallow-water ecosystems along coasts that serve as nurseries for many larger fish, and can be home to thousands of different organisms. Under more acidic lab conditions, they were able to reproduce better, grow taller, and grow deeper roots—all good things. However, they are in decline for a number of other reasons—especially pollution flowing into coastal seawater—and it's unlikely that this boost from acidification will compensate entirely for losses caused by these other stresses.
Some species of algae grow better under more acidic conditions with the boost in carbon dioxide. But coralline algae, which build calcium carbonate skeletons and help cement coral reefs, do not fare so well. Most coralline algae species build shells from the high-magnesium calcite form of calcium carbonate, which is more soluble than the aragonite or regular calcite forms. One study found that, in acidifying conditions, coralline algae covered 92 percent less area, making space for other types of non-calcifying algae, which can smother and damage coral reefs. This is doubly bad because many coral larvae prefer to settle onto coralline algae when they are ready to leave the plankton stage and start life on a coral reef.
One major group of phytoplankton (single celled algae that float and grow in surface waters), the coccolithophores, grows shells. Early studies found that, like other shelled animals, their shells weakened, making them susceptible to damage. But a longer-term study let a common coccolithophore (Emiliania huxleyi) reproduce for 700 generations, taking about 12 full months, in the warmer and more acidic conditions expected to become reality in 100 years. The population was able to adapt, growing strong shells. It could be that they just needed more time to adapt, or that adaptation varies species by species or even population by population.
Fish
While fish don't have shells, they will still feel the effects of acidification. Because the surrounding water has a lower pH, a fish's cells often come into balance with the seawater by taking in carbonic acid. This changes the pH of the fish's blood, a condition called acidosis.
Although the fish is then in harmony with its environment, many of the chemical reactions that take place in its body can be altered. Just a small change in pH can make a huge difference in survival. In humans, for instance, a drop in blood pH of 0.2-0.3 can cause seizures, comas, and even death. Likewise, a fish is also sensitive to pH and has to put its body into overdrive to bring its chemistry back to normal. To do so, it will burn extra energy to excrete the excess acid out of its blood through its gills, kidneys and intestines. It might not seem like this would use a lot of energy, but even a slight increase reduces the energy a fish has to take care of other tasks, such as digesting food, swimming rapidly to escape predators or catch food, and reproducing. It can also slow fishes growth.
Even slightly more acidic water may also affects fishes' minds. While clownfish can normally hear and avoid noisy predators, in more acidic water, they do not flee threatening noise. Clownfish also stray farther from home and have trouble "smelling" their way back. This may happen because acidification, which changes the pH of a fish's body and brain, could alter how the brain processes information. Additionally, cobia (a kind of popular game fish) grow larger otoliths—small ear bones that affect hearing and balance—in more acidic water, which could affect their ability to navigate and avoid prey. While there is still a lot to learn, these findings suggest that we may see unpredictable changes in animal behavior under acidification.
The ability to adapt to higher acidity will vary from fish species to fish species, and what qualities will help or hurt a given fish species is unknown. A shift in dominant fish species could have major impacts on the food web and on human fisheries.
Studying Acidification
In the Past
Geologists study the potential effects of acidification by digging into Earth’s past when ocean carbon dioxide and temperature were similar to conditions found today. One way is to study cores, soil and rock samples taken from the surface to deep in the Earth’s crust, with layers that go back 65 million years. The chemical composition of fossils in cores from the deep ocean show that it’s been 35 million years since the Earth last experienced today’s high levels of atmospheric carbon dioxide. But to predict the future—what the Earth might look like at the end of the century—geologists have to look back another 20 million years.
Some 55.8 million years ago, massive amounts of carbon dioxide were released into the atmosphere, and temperatures rose by about 9°F (5°C), a period known as the Paleocene-Eocene Thermal Maximum. Scientists don’t yet know why this happened, but there are several possibilities: intense volcanic activity, breakdown of ocean sediments, or widespread fires that burned forests, peat, and coal. Like today, the pH of the deep ocean dropped quickly as carbon dioxide rapidly rose, causing a sudden “dissolution event” in which so much of the shelled sea life disappeared that the sediment changed from primarily white calcium carbonate “chalk” to red-brown mud.
Looking even farther back—about 300 million years—geologists see a number of changes that share many of the characteristics of today’s human-driven ocean acidification, including the near-disappearance of coral reefs. However, no past event perfectly mimics the conditions we’re seeing today. The main difference is that, today, CO2 levels are rising at an unprecedented rate—even faster than during the Paleocene-Eocene Thermal Maximum.
In the Lab
Another way to study how marine organisms in today’s ocean might respond to more acidic seawater is to perform controlled laboratory experiments. Researchers will often place organisms in tanks of water with different pH levels to see how they fare and whether they adapt to the conditions. They’re not just looking for shell-building ability; researchers also study their behavior, energy use, immune response and reproductive success. They also look at different life stages of the same species because sometimes an adult will easily adapt, but young larvae will not—or vice versa. Studying the effects of acidification with other stressors such as warming and pollution, is also important, since acidification is not the only way that humans are changing the oceans.
In the wild, however, those algae, plants, and animals are not living in isolation: they’re part of communities of many organisms. So some researchers have looked at the effects of acidification on the interactions between species in the lab, often between prey and predator. Results can be complex. In more acidic seawater, a snail called the common periwinkle (Littorina littorea) builds a weaker shell and avoids crab predators—but in the process, may also spend less time looking for food. Boring sponges drill into coral skeletons and scallop shells more quickly. And the late-stage larvae of black-finned clownfish lose their ability to smell the difference between predators and non-predators, even becoming attracted to predators.
Although the current rate of ocean acidification is higher than during past (natural) events, it’s still not happening all at once. So short-term studies of acidification’s effects might not uncover the potential for some populations or species to acclimate to or adapt to decreasing ocean pH. For example, the deepwater coral Lophelia pertusa shows a significant decline in its ability to maintain its calcium-carbonate skeleton during the first week of exposure to decreased pH. But after six months in acidified seawater, the coral had adjusted to the new conditions and returned to a normal growth rate.
Natural Variation
There are places scattered throughout the ocean where cool CO2-rich water bubbles from volcanic vents, lowering the pH in surrounding waters. Scientists study these unusual communities for clues to what an acidified ocean will look like.
Researchers working off the Italian coast compared the ability of 79 species of bottom-dwelling invertebrates to settle in areas at different distances from CO2 vents. For most species, including worms, mollusks, and crustaceans, the closer to the vent (and the more acidic the water), the fewer the number of individuals that were able to colonize or survive. Algae and animals that need abundant calcium-carbonate, like reef-building corals, snails, barnacles, sea urchins, and coralline algae, were absent or much less abundant in acidified water, which were dominated by dense stands of sea grass and brown algae. Only one species, the polychaete worm Syllis prolifers, was more abundant in lower pH water. The effects of carbon dioxide seeps on a coral reef in Papua New Guinea were also dramatic, with large boulder corals replacing complex branching forms and, in some places, with sand, rubble and algae beds replacing corals entirely.
All of these studies provide strong evidence that an acidified ocean will look quite different from today’s ocean. Some species will soldier on while others will decrease or go extinct—and altogether the ocean’s various habitats will no longer provide the diversity we depend on.
Field Experiments
One challenge of studying acidification in the lab is that you can only really look at a couple species at a time. To study whole ecosystems—including the many other environmental effects beyond acidification, including warming, pollution, and overfishing—scientists need to do it in the field.
The biggest field experiment underway studying acidification is the Biological Impacts of Ocean Acidification (BIOACID) project. Scientists from five European countries built ten mesocosms—essentially giant test tubes 60-feet deep that hold almost 15,000 gallons of water—and placed them in the Swedish Gullmar Fjord. After letting plankton and other tiny organisms drift or swim in, the researchers sealed the test tubes and decreased the pH to 7.8, the expected acidity for 2100, in half of them. Now they are waiting to see how the organisms will react, and whether they're able to adapt. If this experiment, one of the first of its kind, is successful, it can be repeated in different ocean areas around the world.
Looking to the Future
If the amount of carbon dioxide in the atmosphere stabilizes, eventually buffering (or neutralizing) will occur and pH will return to normal. This is why there are periods in the past with much higher levels of carbon dioxide but no evidence of ocean acidification: the rate of carbon dioxide increase was slower, so the ocean had time to buffer and adapt. But this time, pH is dropping too quickly. Buffering will take thousands of years, which is way too long a period of time for the ocean organisms affected now and in the near future.
So far, the signs of acidification visible to humans are few. But they will only increase as more carbon dioxide dissolves into seawater over time. What can we do to stop it?
Cut Carbon Emissions
In 2013, carbon dioxide in the atmosphere passed 400 parts per million (ppm)—higher than at any time in the last one million years (and maybe even 25 million years). The "safe" level of carbon dioxide is around 350 ppm, a milestone we passed in 1988. Without ocean absorption, atmospheric carbon dioxide would be even higher—closer to 475 ppm.
The most realistic way to lower this number—or to keep it from getting astronomically higher—would be to reduce our carbon emissions by burning less fossil fuels and finding more carbon sinks, such as regrowing mangroves, seagrass beds, and marshes, known as blue carbon. If we did, over hundreds of thousands of years, carbon dioxide in the atmosphere and ocean would stabilize again.
Even if we stopped emitting all carbon right now, ocean acidification would not end immediately. This is because there is a lag between changing our emissions and when we start to feel the effects. It's kind of like making a short stop while driving a car: even if you slam the brakes, the car will still move for tens or hundreds of feet before coming to a halt. The same thing happens with emissions, but instead of stopping a moving vehicle, the climate will continue to change, the atmosphere will continue to warm and the ocean will continue to acidify. Carbon dioxide typically lasts in the atmosphere for hundreds of years; in the ocean, this effect is amplified further as more acidic ocean waters mix with deep water over a cycle that also lasts hundreds of years.
Geoengineering
It's possible that we will develop technologies that can help us reduce atmospheric carbon dioxide or the acidity of the ocean more quickly or without needing to cut carbon emissions very drastically. Because such solutions would require us to deliberately manipulate planetary systems and the biosphere (whether through the atmosphere, ocean, or other natural systems), such solutions are grouped under the title "geoengineering."
The main effect of increasing carbon dioxide that weighs on people's minds is the warming of the planet. Some geoengineering proposals address this through various ways of reflecting sunlight—and thus excess heat—back into space from the atmosphere. This could be done by releasing particles into the high atmosphere, which act like tiny, reflecting mirrors, or even by putting giant reflecting mirrors in orbit! However, this solution does nothing to remove carbon dioxide from the atmosphere, and this carbon dioxide would continue to dissolve into the ocean and cause acidification.
Another idea is to remove carbon dioxide from the atmosphere by growing more of the organisms that use it up: phytoplankton. Adding iron or other fertilizers to the ocean could cause man-made phytoplankton blooms. This phytoplankton would then absorb carbon dioxide from the atmosphere, and then, after death, sink down and trap it in the deep sea. However, it's unknown how this would affect marine food webs that depend on phytoplankton, or whether this would just cause the deep sea to become more acidic itself.
What You Can Do
Even though the ocean may seem far away from your front door, there are things you can do in your life and in your home that can help to slow ocean acidification and carbon dioxide emissions.
The best thing you can do is to try and lower how much carbon dioxide you use every day. Try to reduce your energy use at home by recycling, turning off unused lights, walking or biking short distances instead of driving, using public transportation, and supporting clean energy, such as solar, wind, and geothermal power. Even the simple act of checking your tire pressure (or asking your parents to check theirs) can lower gas consumption and reduce your carbon footprint. (Calculate your carbon footprint here.)
One of the most important things you can do is to tell your friends and family about ocean acidification. Because scientists only noticed what a big problem it is fairly recently, a lot of people still don't know it is happening. So talk about it! Educate your classmates, coworkers and friends about how acidification will affect the amazing ocean animals that provide food, income, and beauty to billions of people around the world.
Additional Resources
NOAA Ocean Acidification Program
What is Ocean Acidification? - NOAA Pacific Marine Environmental Laboratory (PMEL) Carbon Program
Impacts of Ocean Acidification - European Science Foundation
Covering Ocean Acidification: Chemistry and Considerations - Yale Climate Media Forum
An Introduction to the Chemistry of Ocean Acidification - Skeptical Science
Frequently Asked Questions about Ocean Acidification - BIOACID
Ocean Acidification at Point Reyes National Seashore (Video) - National Park Service
News Articles
Sea Change (Seattle Times)
Bad acid trip: A beach bum’s guide to ocean acidification (Grist)
What Does Ocean Acidification Mean for Sea Life? (Ensia)
10 Key Findings From a Rapidly Acidifying Arctic Ocean (Mother Jones)
Scientific Papers
Ocean Acidification and Its Potential Effects on Marine Ecosystems - John Guinotte & Victoria Fabry
Impacts of ocean acidification on marine fauna and ecosystem processes - Victoria Fabry, Brad Seibel, Richard Feely, & James Orr